Handling your study
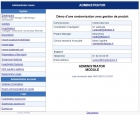
For a perfect autonomy in the management of your study, EOL© has a management console to configure and administer your study.
The main features of the console are:
- To manage Centres;
- To manage Investigators, CSTs, Study Nurses, CRAs, Project Managers, Sponsors;
- To manage authorizations;
- To assign users to roles by creating conditions for their access to eCRF;
- To re-send their IDs to users;
- To import entries made on other systems;
- To visualise connection logs and administration actions;
- To update the documents attached to the study (Inform Consent, Protocol, Quality of Life Questionnaires,...);
- Manage the email addresses of users;
- Change some general parameters of the study (name/phone of contacts, start and end of study ...).
Our news
EOL New brochure available!
March 2016 : EOL the new brochure is available!
Medsharing in figures
(Figures 2015)
78,000 patients since 2003 for over 130 studies.
26 Registries / Observatories.
49 Studies.
61 Clinical Trials
31 Randomizations (without eCRF)
47 Drug Studies
28 DM Studies
66 Ongoing studies.
Figures 2015
New version of the application of randomization
Check out the new unlimited version of our Randomization for clinical trial application IPHONE / IPAD.
Quote request
+33 (0)1 48 75 39 14
by e-mail