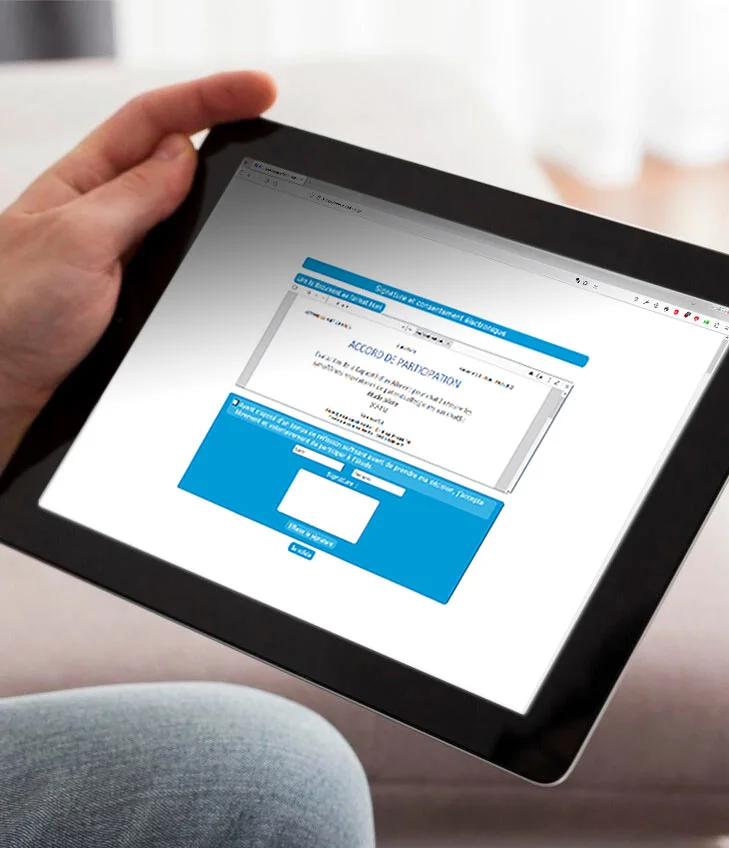
eConsent (electronic consent) allows the investigator physician to send a consent request directly via the eCRF before enrolling the patient in the study.
Alone or integrated into the eCRF, eConsent simplifies the monitor’s work while ensuring data security and confidentiality :
- Procedural compliance : patients cannot be enrolled until they have signed,
- Simplicity : a unique code links the patient record to the consent,
- Remote monitoring : the monitor can oversee the process without traveling to the site,
- Confidentiality : remotely, the monitor has no access to personal data,
- Security : personal data are encrypted; consents are password-protected,
- Traceability : a complete audit trail of the procedure is printed on each document.
The eConsent signature in EOL is based on a “simple level” signature according to eIDAS (Regulation No. 910/2014/EU, adopted July 23, 2014) and uses an OTP (One-Time Password) system..