A digital tool serving quality, efficiency, and compliance.
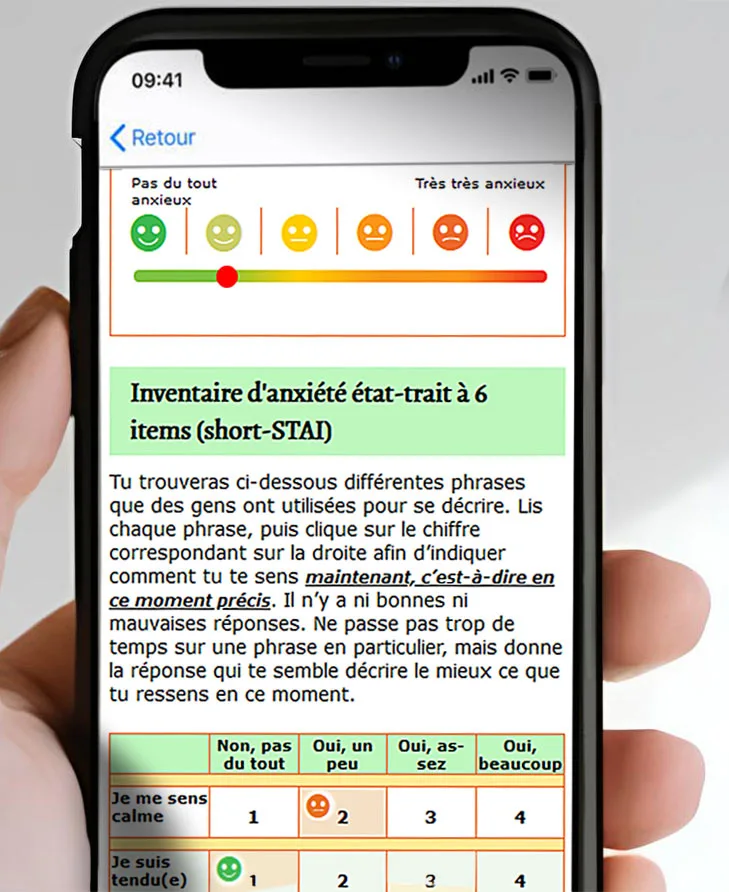
ePRO (electronic Patient-Reported Outcome) has become an essential solution for directly collecting patient data in clinical trials. It offers a modern, reliable, and compliant approach that transforms the participant experience while optimizing study conduct.
Key advantages of ePRO :
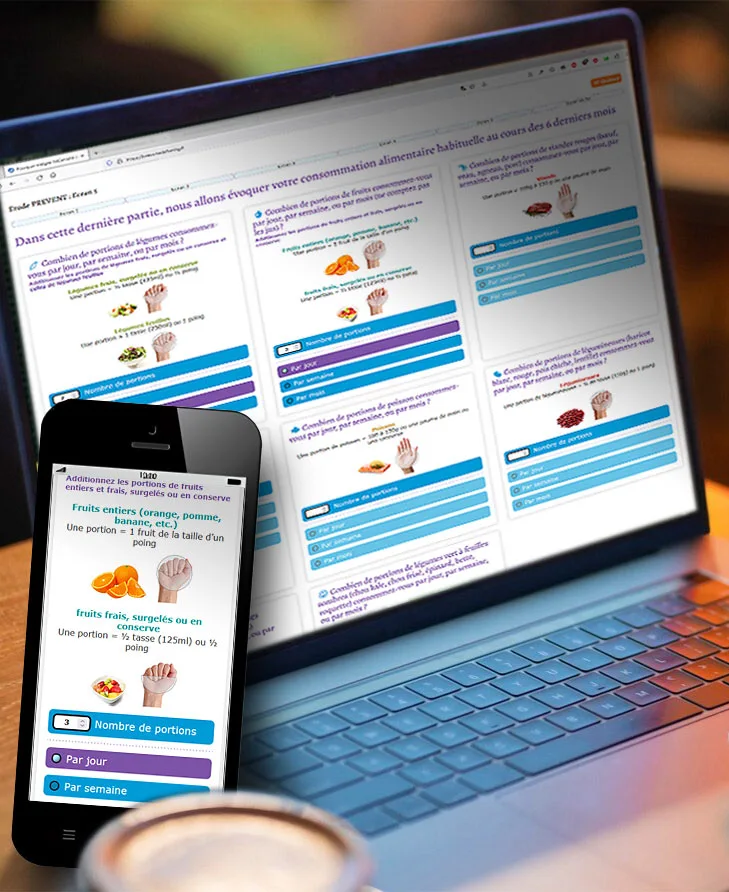
- Simplified data collection : Participants enter responses directly on smartphone, tablet, or computer. Eliminates paper use, reducing errors and processing delays,
- L'ePRO & eCOA – comprehensive data coverage : ePRO fits within the broader eCOA (electronic Clinical Outcome Assessment) framework, encompassing all electronically reported outcomes from patients, caregivers, or clinicians—resulting in higher data quality and reliability,
- Enhanced experience & cost reduction : ePRO lightens logistical burdens for participants and investigators. Fewer site visits and manual re-entries lead to significant study-cost savings,
- Stronger compliance & error detection : Built-in consistency checks catch discrepancies in real time. ePRO also supports regulatory compliance with full audit-trail traceability and robust security,
- Data security guaranteed : Access is strictly controlled by role- and rights-management systems. Only authorized users can view or edit data, ensuring integrity and confidentiality,
- Facilitated collaboration & advanced features : ePRO supports multi-user entry and offers advanced capabilities :
flexible form creation, automated reminders, real-time dashboards... for optimal study management..